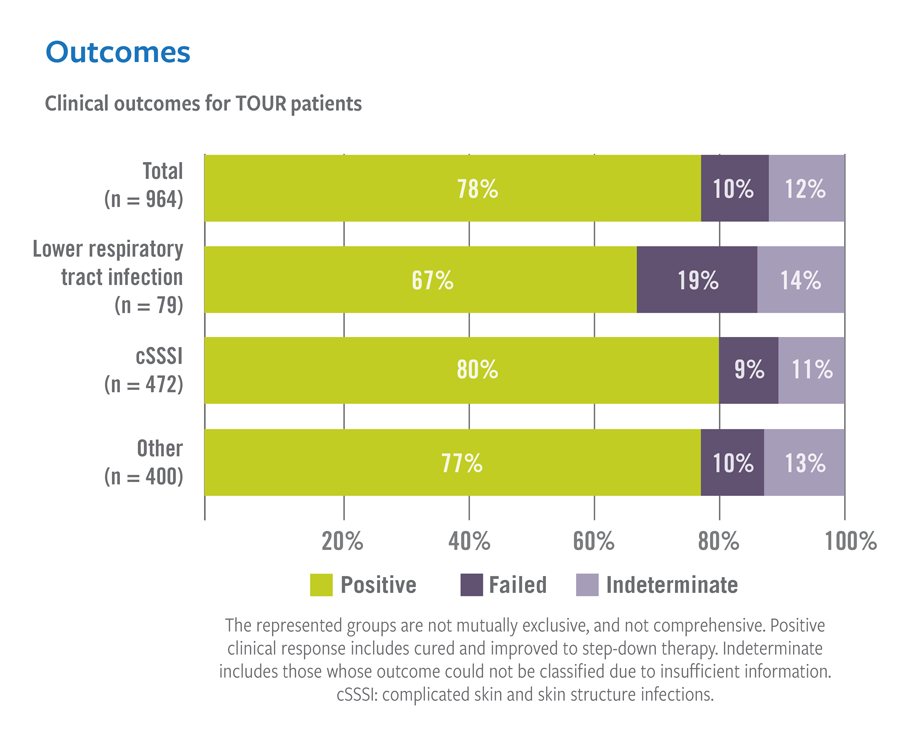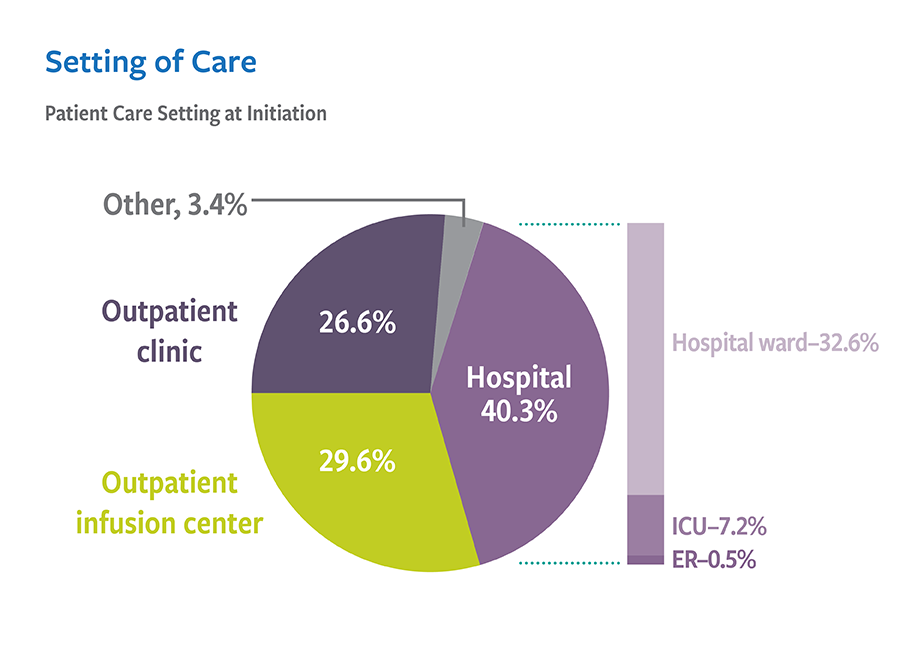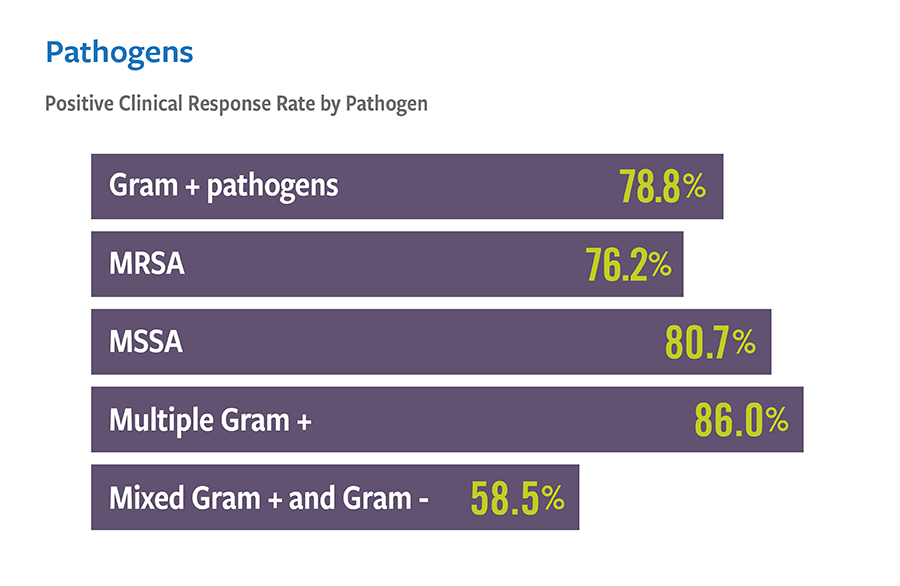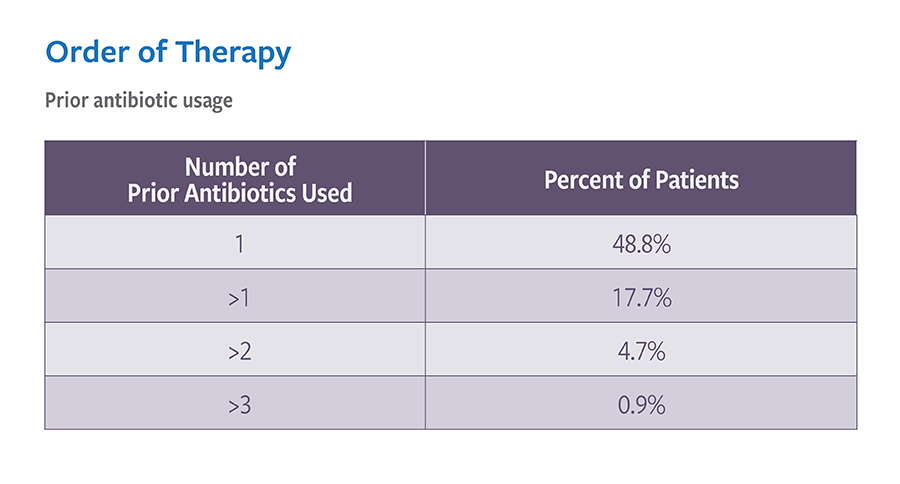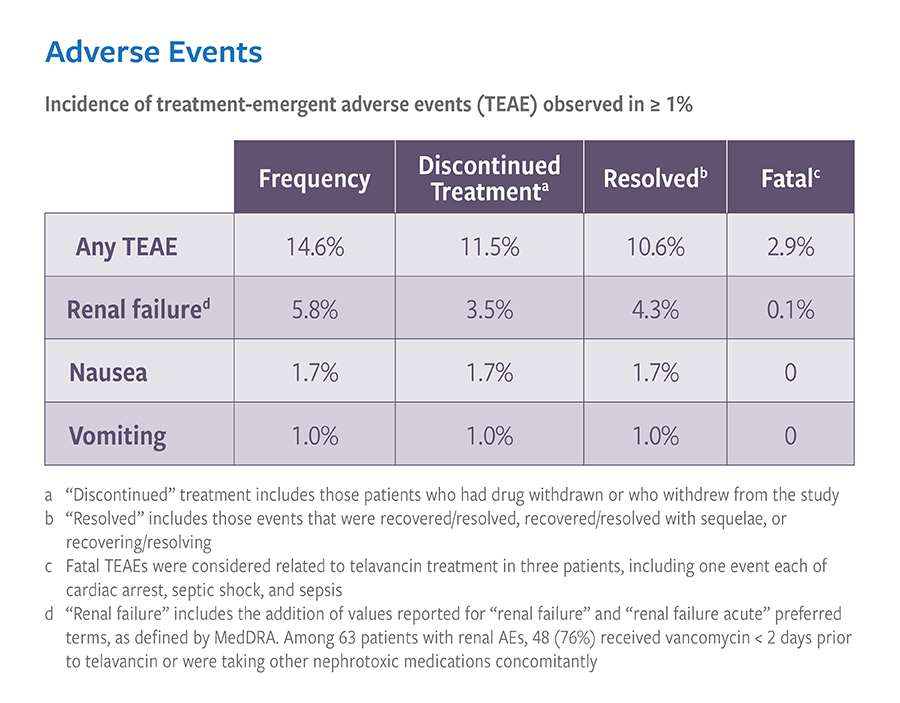
Real-world treatment results support the use of VIBATIV for a variety of infections due to Gram-positive pathogens. The Telavancin Observational Use Registry (TOUR™) recorded characteristics and outcomes of telavancin use in clinical practice. TOUR includes data collected from 45 US sites for 1,063 patients treated between January 2015–March 2017. Clinical outcomes for TOUR patients available for assessment at the end of treatment for major infection types were assessed. The represented groups are not mutually exclusive, and not comprehensive as Telavancin therapy was used at the discretion of the attending study physician and not the registry study.