To learn how VIBATIV may help treat your patients with HABP/VABP or cSSSI, use the tool below.
Think about a patient you’re currently treating.
Patient with HABP and vancomycin MIC ≥1 μg/mL

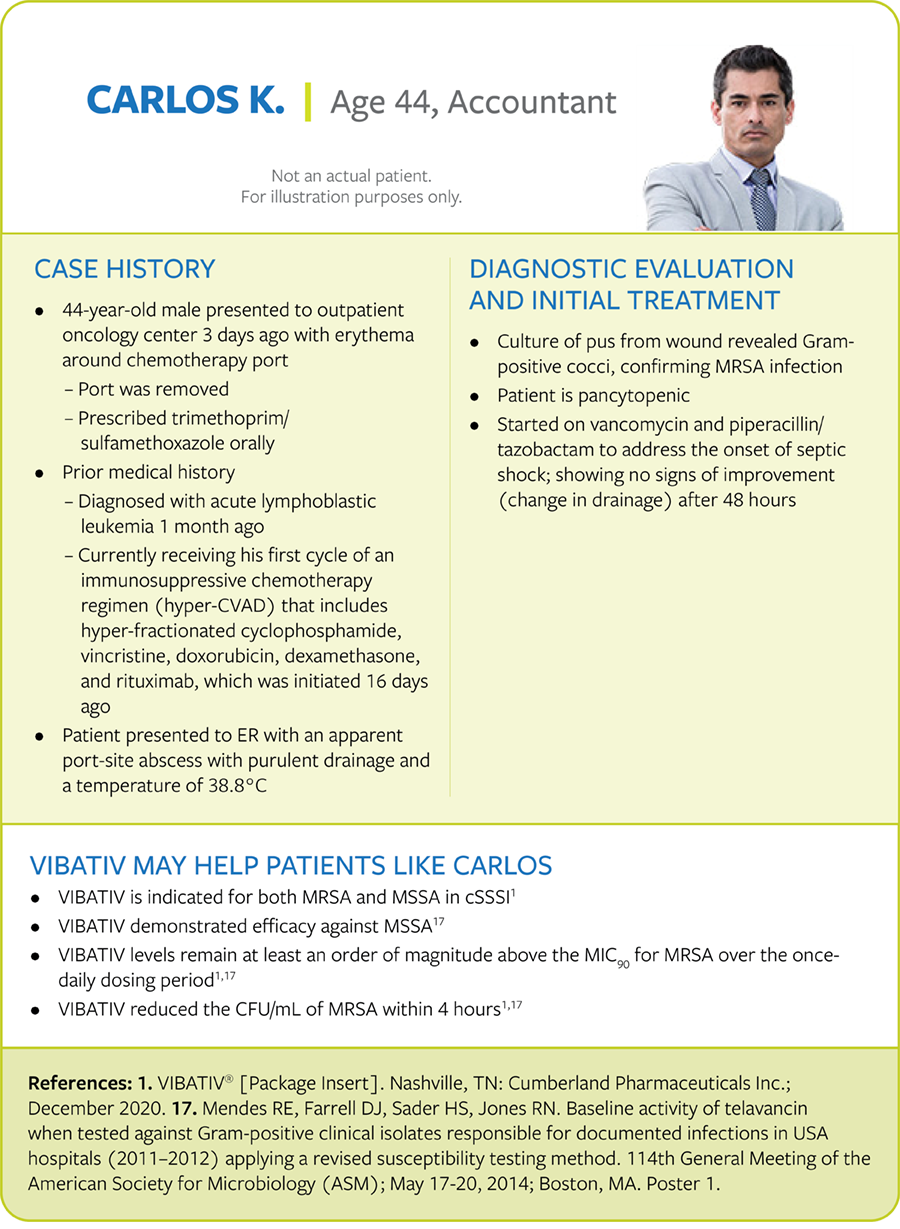
Immunocompromised patient with cSSSI

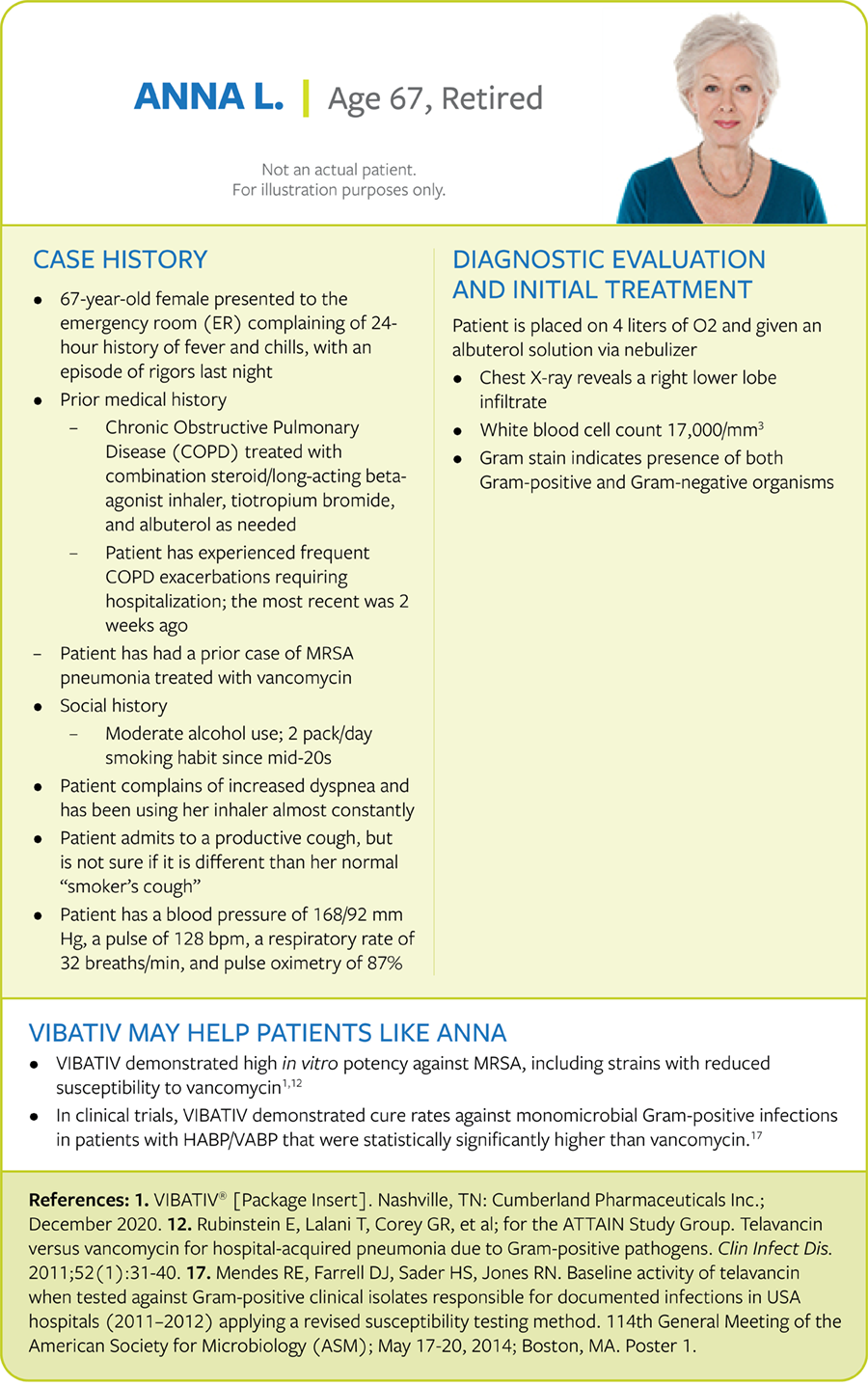
Elderly patient with HABP due to a recurrent S. aureus infection

Patient with VABP requiring <7 days of therapy

Patient with cSSSI who is able to transition to outpatient treatment

Patient with HABP taking an SSRI and hypoglycemic agents for type 2 diabetes

Patient with VABP and complicating factors

Patient with cSSSI and recurrent S. aureus infection

Patient with HABP who requires high doses of vancomycin

Patient with HABP and chronic obstructive pulmonary disease (COPD)

Overweight patient with cSSSI who requires an IV antibiotic